To recap, Allena has made some pretty big changes to their Phase 3 design, compared to previous trials. Urirox-1 is being tested in enteric hyperoxaluria patients only, have increased the dose to match the patient’s eating habits, are using a Time Weighted Average to measure UOx reduction, and are raising the baseline UOx levels to above 50 mg/day. Is this enough to reduce the large variability we have seen in the past?
Cons
–Contribution of diet: What’s clear is that diet affects UOx levels. What’s not clear is by how much. Some studies state the contribution is 10-20% (Williams 1989), while others show up to 41.5% (Holmes 2001). Calcium content and hydration status can also lower UOx levels.
–Is 20% enough?: Mouse studies of OxDc-CLEC only showed an increased survival at 40% reduction of UOx. There was not a clear dose response at doses below that level, though all doses lowered UOx. Is the 20% bar ALNA is aiming for enough to show lowering of kidney stone formation? This will be answered in the second Phase 3 trial, Urirox-2.
–Why the 50 mg/day cutoff?: While the higher baseline patients historically respond better, why did ALNA choose 50 mg/day as the cutoff? The company has stated they consider that a “severe” population, yet their average baseline is 103 mg/day (making that super severe?). I can not find any clear clinical reasoning behind 50 mg/day cutoff, aside from ALNA noticing Reloxaliase has a better effect above these levels.
–Oxazyme no longer being pursued: Oxazyme (OC4) is a similar enzyme to Reloxaliase. However, the compound is no longer being pursued in favor of a bacteria therapeutic. More on Oxazyme below.
–How high are EH UOx levels actually?: Per the company, 44.4% of enteric population had at least 1 normal UOx value <45 mg/day over 22.5 months, although overall UOx was higher. About 40% of idiopathic patients also approached absorption levels of enteric patients, but Reloxaliase had a dramatically worse effect in IH.
–Effect of the microbiome?: Several reports have shown that Oxalobacter formigenes can degrade oxalate. Consistent with other microbiome trials, studies of O. formigenes have been…inconsistent. However, there is some evidence that hyperoxaluria patients have lower levels of O. formigenes (Nazzal 2015).
–Potential placebo response?: A few placebo enteric patients at high UOx baseline have shown drastic lowering of UOx. The company expects no placebo response, overall.

–Variable measurements: It was found that peak UOx occurs 2-4 hours after oxalate intake. Clearly, the timing of capturing UOx levels makes a difference. Using a time weighted average will seek to address this.
–Suboptimal pH: OxDc has optimal activity in acidic pHs, but down to about 2-7% residual activity at neutral pHs (Conter 2019).
–What happened in Study 649?: Recall, this crossover trial of two 7-day treatment periods was stopped early due to no difference w/placebo. However, other trials as short as 4 days showed an effect on UOx. Is this due to inconsistency in drug efficacy, or an issue with the crossover design, which theoretically reduces inter-patient variability, but not intra-patient.
Pros
–Time Weighted Average: TWA is defined as the average of all 24 hour UOx excretion values while obtained on drug, with each value weighted for the number of days since the last urine collection. In theory, this would lower variability in UOx by accounting for differences in hydration status, inherent variability with the assay, dietary changes, and sample handling.
–Cutoff is more stringent than meets the eye: A previous Phase 2 study had a 50 mg/day UOx cutoff. However, in that trial, the patients only needed to have a > 50 mg/day reading at screen-in. In Urirox-1, patients must have both a >50 mg/day at screen-in and two baseline measurements above 50 mg/day. This should add some consistency to the results
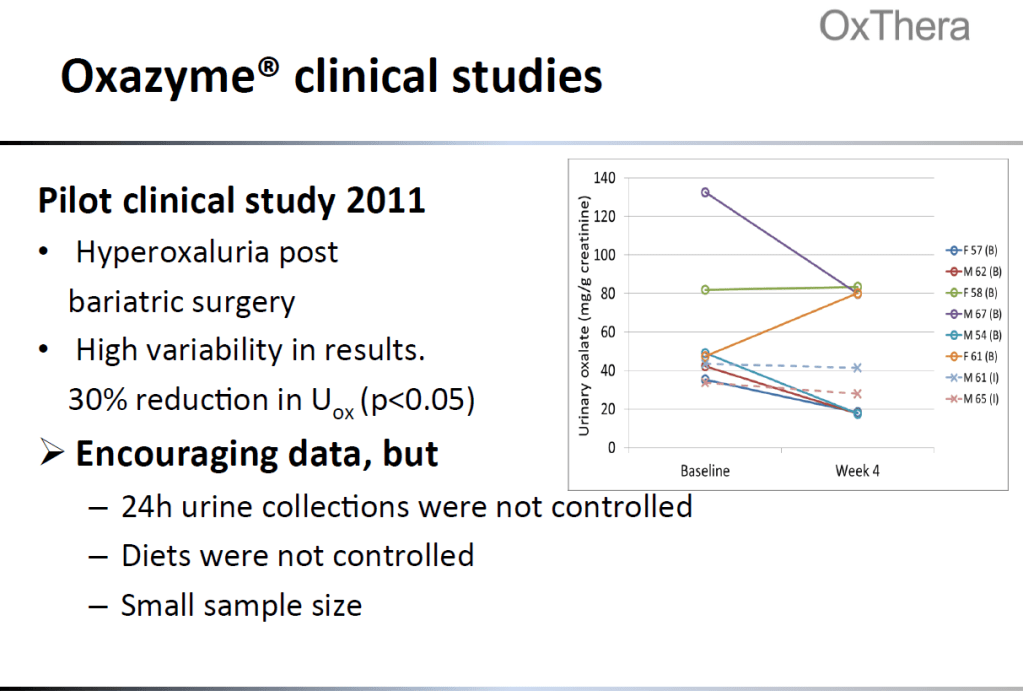
–Proof of concept?: Oxazyme has shown some efficacy in a small trial. Data from the Oxazyme trial is below. Nephure is also an enzyme dietary supplement designed to lower oxalate (and has multiple 5 star reviews on Amazon! Some customers have lab tests showing efficacy. “Stephen” left a useless 3 star review saying he doesn’t know if it works yet. Why leave a review then!?!)
-UOx is an accepted endpoint: The FDA has agreed that UOx lowering is an acceptable surrogate endpoint for kidney stone progression (ALNA will look to confirm this in Urirox-2). Of note, time weighted averages have also been used historically (ex. Tenofovir)
–EH and higher UOx: Though the UOx is variable among enteric patients, with some reporting occasional normal values, the overall population has a higher UOx level than other secondary hyperoxaluria patients. This would be the population to show the most benefit, if Reloxaliase works as intended.
Misc.
-Oxazyme is similar to Reloxaliase, but with one amino acid swapped to prevent aggregation. It was tried in an enteric population (post-bariatric surgery), but also showed high variability in UOx reduction, though the overall reduction was by 30%. Oxazyme has been tabled in favor of an Oxalobacter formigenes product.
-Nephure is being marketed as a dietary supplement to lower oxalate levels. Like Reloxaliase, Nephure is a powder form to be taken with meals. A white paper published by the company showed an average reduction of 13 mg/day in normal patients on high oxalate diets. However, normal patients and hyperoxaluria patients respond different to oxalate intake. Now marketed, some anecdotes with lab results from customers show some lowering of UOx.
-Cash levels will be ~$40m at the end of 3Q19, equating to $1.70/sh

